Market Outlook
The US Implantable Cardioverter Defibrillator (ICD) Devices Market was valued at USD 4.62 billion in 2025 and is projected to reach approximately USD 8.94 billion by 2035, expanding at a CAGR of 6.82% during the forecast period 2026–2035. Historical analysis has been conducted from 2021 to 2024, capturing procedural volumes, reimbursement trends, and evolving device innovation across leading U.S. cardiac centers.
Implantable cardioverter defibrillators are life-saving cardiac rhythm management devices designed to detect and terminate life-threatening ventricular arrhythmias. In the United States, cardiovascular disease remains the leading cause of mortality, accounting for nearly one in every five deaths. Sudden cardiac arrest continues to pose a substantial public health burden, particularly among aging populations and patients with ischemic heart disease or cardiomyopathy. ICD therapy is clinically established as a gold standard for both primary and secondary prevention of sudden cardiac death.
The U.S. market benefits from advanced electrophysiology infrastructure, high healthcare spending, favorable reimbursement under Medicare and private insurers, and strong adoption of technologically advanced subcutaneous and MRI-compatible devices. Increasing emphasis on remote monitoring, AI-integrated arrhythmia detection, and minimally invasive implantation procedures is reshaping the competitive landscape.
Introduction
According to this US Implantable Cardioverter Defibrillator Devices Market report, the sector reflects steady growth driven by epidemiological burden, technological innovation, and established reimbursement pathways. ICD devices function by continuously monitoring heart rhythm and delivering electrical therapy when abnormal rhythms such as ventricular tachycardia or ventricular fibrillation are detected. In many cases, ICD therapy significantly reduces mortality risk in high-risk cardiac patients.
In the United States, increasing prevalence of heart failure, coronary artery disease, diabetes, and hypertension are creating sustained demand for ICD implantation. Additionally, the expansion of electrophysiology labs, ambulatory surgical capabilities, and remote monitoring networks is improving patient access. Regulatory oversight by the U.S. Food and Drug Administration ensures high clinical safety standards, further strengthening physician confidence in ICD therapy.
As healthcare systems prioritize value-based care, ICD technology has evolved toward longer battery life, reduced complication rates, and improved patient quality of life. The integration of wireless data transmission and smartphone-based monitoring tools is transforming post-implant care management.
Key Market Drivers: What’s Fueling the US ICD Devices Market Growth?
- Rising Incidence of Sudden Cardiac Arrest
Sudden cardiac arrest affects more than 350,000 individuals annually in the United States. Patients with prior myocardial infarction or left ventricular dysfunction are at significantly elevated risk. ICD implantation has demonstrated mortality reduction of up to 30% in high-risk populations. This strong clinical evidence base continues to drive adoption across cardiology practices nationwide. - Growing Geriatric Population
The U.S. population aged 65 years and above is expanding rapidly. Older adults exhibit higher prevalence of heart failure and arrhythmias, increasing candidacy for ICD therapy. By 2035, seniors are expected to represent more than 20% of the total U.S. population, reinforcing long-term device demand. - Technological Advancements in Device Design
Subcutaneous ICDs (S-ICDs) reduce complications associated with transvenous leads, while MRI-compatible devices improve diagnostic flexibility. Battery longevity improvements now extend device life beyond 10 years in many cases. These advancements enhance patient outcomes and physician adoption. - Expansion of Remote Monitoring
Remote cardiac monitoring platforms reduce hospital visits, enable early detection of device or rhythm issues, and align with U.S. telehealth expansion policies. Reimbursement coverage for remote device management supports consistent revenue streams for healthcare providers.
Recent Developments
Recent developments include expanded FDA approvals for MRI-safe ICD models, AI-enabled arrhythmia detection algorithms, and remote monitoring software upgrades. Several manufacturers have introduced devices with extended battery life and improved lead durability. Partnerships between device companies and digital health platforms are enhancing data integration and patient engagement.
Conclusion
The US Implantable Cardioverter Defibrillator Devices Market demonstrates stable, innovation-driven growth supported by strong clinical evidence, favorable reimbursement frameworks, and advanced electrophysiology infrastructure. Rising cardiovascular disease burden and expanding geriatric population ensure sustained procedural demand through 2035. Technological evolution toward minimally invasive, connected, and durable devices will shape competitive differentiation.
With continued investments in remote monitoring, digital integration, and patient-centric therapy design, the market is positioned for long-term resilience and incremental expansion across all major U.S. regions.
Key Market Players
The US Implantable Cardioverter Defibrillator Devices Market is moderately consolidated, with major multinational corporations leading the space alongside select niche device manufacturers.
- Medtronic – U.S. leader in transvenous and CRT-D technologies with strong hospital penetration.
- Abbott Laboratories – Offers advanced MRI-compatible ICD platforms widely adopted across U.S. centers.
- Boston Scientific Corporation – Pioneer in subcutaneous ICD systems with strong domestic distribution.
- Biotronik – Expanding presence in U.S. electrophysiology labs.
- MicroPort CRM – Growing U.S. footprint with innovative cardiac rhythm solutions.
- LivaNova – Active in cardiac rhythm technologies and heart failure solutions.
- ZOLL Medical Corporation – Known for external defibrillation technologies and cardiac support systems.
- Philips Healthcare – Provides monitoring systems supporting ICD management.
- GE HealthCare – Integrates imaging with electrophysiology services.
- Siemens Healthineers – Supports cardiac diagnostic infrastructure.
- BIOTRONIK USA – U.S.-focused expansion of implantable rhythm devices.
- Cardiac Science – Provides cardiac emergency solutions.
- Nihon Kohden America – Monitoring systems supporting ICD patients.
- Progetti Medical – Expanding defibrillator technologies.
- Sorin Group – Historical presence in cardiac rhythm devices.
- Shree Pacetronix – Emerging global ICD manufacturer exploring U.S. entry.
- Osypka Medical – Specialty cardiac pacing products.
- Vitatron – Known for pacemaker technologies influencing ICD innovations.
- Vectorious Medical – Remote heart monitoring innovation.
- Kestra Medical Technologies – Cardiac wearable defibrillator technology developer.
- Cameron Health (acquired by Boston Scientific) – Developer of early S-ICD technology.
- CardioFocus – Supports electrophysiology ecosystem.
- Transvenous Implantable Cardioverter Defibrillators (Dominant Segment)
Transvenous ICDs represent the majority of U.S. implant volumes. These devices include leads placed within the heart via venous access and are widely used for primary and secondary prevention. Their long-standing clinical history and familiarity among electrophysiologists contribute to market dominance. - Subcutaneous Implantable Cardioverter Defibrillators (Fastest-Growing Segment)
S-ICDs eliminate intracardiac leads, reducing risks of vascular complications. Adoption is increasing in younger patients and those with challenging venous access. Growing awareness of long-term lead complications supports continued growth. - Cardiac Resynchronization Therapy Defibrillators (CRT-D)
CRT-D devices combine pacing therapy with defibrillation and are commonly used in patients with advanced heart failure and conduction abnormalities. These devices hold significant value in tertiary care hospitals.
- Pulse Generators
Pulse generators represent the largest revenue share due to higher unit cost and technological sophistication. Continuous innovation in shock delivery efficiency and arrhythmia detection algorithms supports growth. - Leads
Leads remain critical components but are associated with complication risks. Manufacturers are investing in improved insulation materials and durability enhancements to minimize revisions. - Batteries & Accessories
Battery technology is evolving to extend device longevity and reduce replacement surgeries. Accessories such as programmers and home monitoring transmitters support recurring revenue.
- Hospitals (Dominant)
Large academic and tertiary hospitals perform the majority of ICD implantations in the U.S., supported by advanced cath labs and electrophysiology specialists. - Ambulatory Surgical Centers
Outpatient implantation procedures are increasing due to cost efficiency and improved procedural techniques. - Cardiac Specialty Clinics
Private cardiology practices are expanding device follow-up services, particularly through remote monitoring platforms.
- Ventricular Tachycardia
This segment accounts for a significant share, as sustained ventricular tachycardia presents high mortality risk. - Ventricular Fibrillation
ICDs provide immediate life-saving shock therapy in these cases, making this indication central to device utilization. - Heart Failure with Reduced Ejection Fraction
Primary prevention in heart failure patients contributes to steady implantation volumes. - Others
Includes inherited arrhythmia syndromes and structural heart diseases.
West Region (Largest Market Share)
The West region dominates due to advanced healthcare systems and high cardiovascular screening rates.
California leads implantation volumes, supported by major academic centers in Los Angeles and San Francisco. The state accounts for a substantial share of total U.S. cardiac procedures.
Washington benefits from strong healthcare innovation hubs and high per capita healthcare spending.
Colorado and Arizona show rising implantation rates due to aging populations.
Oregon and Nevada demonstrate growing demand driven by expanding hospital networks.
Other states including Utah, Idaho, Montana, Wyoming, Alaska, and Hawaii maintain smaller but steadily growing markets.
Northeast Region (High Technology Adoption)
New York remains a major contributor, supported by leading cardiac hospitals and high procedural capacity.
Massachusetts benefits from research-driven healthcare institutions integrating advanced ICD technologies.
New Jersey and Pennsylvania show strong Medicare-covered implantation volumes.
Connecticut and other states including Maine, Vermont, New Hampshire, Rhode Island, and Delaware exhibit stable growth.
South Region (Fastest-Growing Region)
Texas leads in procedure volume due to large population base and strong hospital networks.
Florida demonstrates high ICD utilization due to its large elderly demographic.
Georgia and North Carolina are investing heavily in cardiac specialty centers.
Tennessee, South Carolina, and Alabama show rising implantation rates as rural cardiac programs expand.
Other states including Mississippi, Louisiana, Arkansas, Kentucky, Oklahoma, Virginia, Maryland, and West Virginia are gradually increasing electrophysiology capacity.
Midwest Region (Steady Growth)
Illinois and Ohio are major ICD implantation hubs with established cardiac centers.
Michigan and Minnesota demonstrate strong academic hospital presence.
Indiana, Wisconsin, and Missouri contribute consistent procedure volumes.
Other states including Iowa, Kansas, Nebraska, North Dakota, and South Dakota show moderate but improving access.
1. Market Introduction & Context
1.1 Market Definition
1.2 Scope of the Study
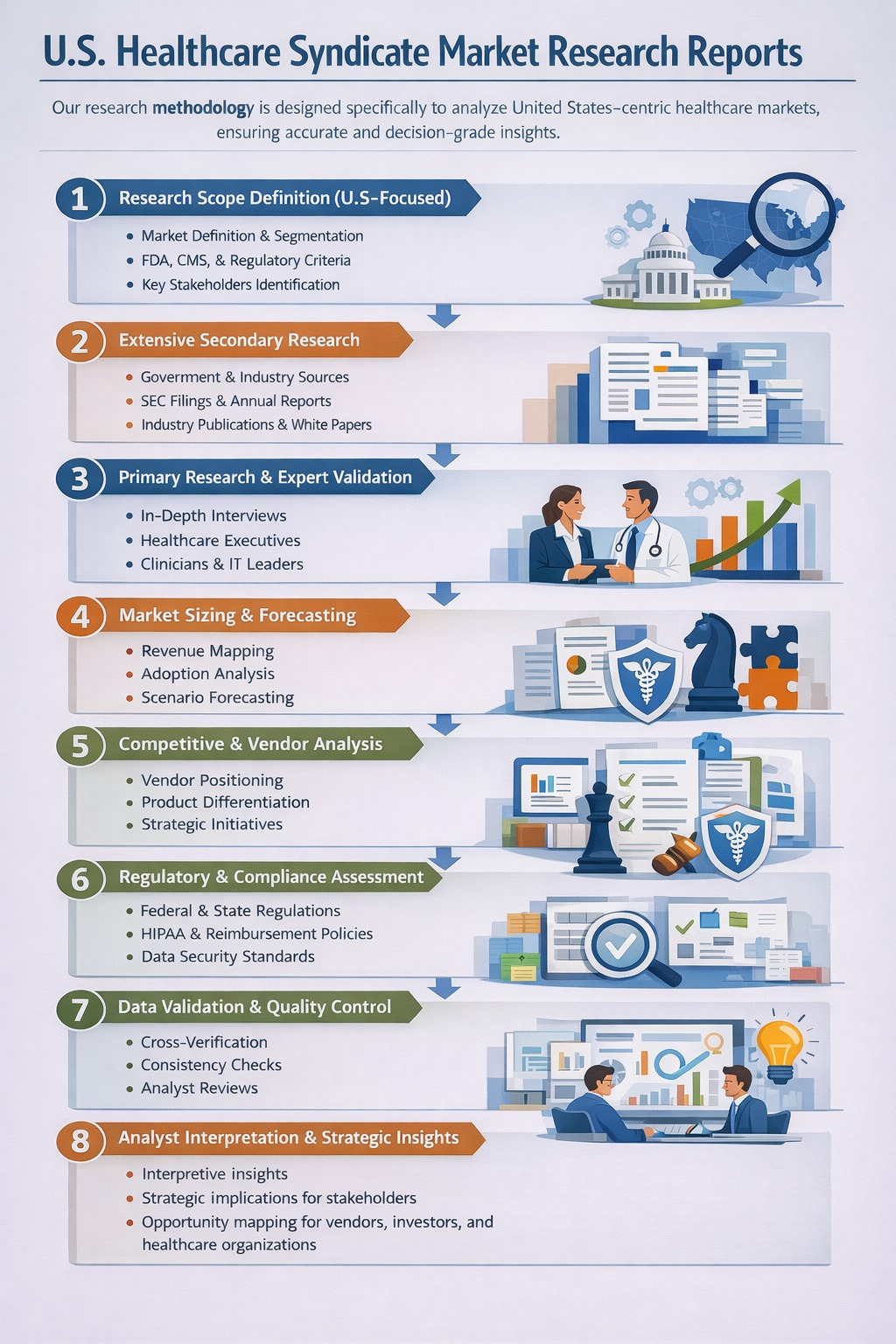
1.3 Research Methodology
1.3.1 Primary Data Collection
1.3.2 Secondary Data Sourcing
1.3.3 External Industry Collaborations
1.3.4 In-House Research Databases
1.3.5 Analytical Frameworks & Forecasting Models
1.3.6 Data Validation and Final Review
1.4 Key Assumptions
1.5 Market Ecosystem Overview
1.6 Stakeholder Analysis
(What this section provides: Overview of study framework, research methodology, validation models, and ecosystem participants ensuring data transparency and reliability.)
2. Executive Summary
2.1 Key Market Highlights
2.2 Analyst Insights
2.3 Market Attractiveness Analysis
(What this section provides: Concise overview of market size, CAGR, segment leadership, and regional performance trends.)
3. Market Dynamics & Outlook
3.1 Market Drivers
3.2 Market Restraints
3.3 Market Opportunities
3.4 Innovation & Patent Landscape (2020–2025)
(What this section provides: Detailed assessment of growth drivers, regulatory trends, reimbursement policies, and innovation developments shaping the ICD market.)
4. Market Environment & Industry Analysis
4.1 PESTEL Analysis
4.1.1 Political
4.1.2 Economic
4.1.3 Social
4.1.4 Technological
4.1.5 Environmental
4.1.6 Legal
4.2 Porter’s Five Forces Analysis
4.3 Pricing Trend Analysis (2024–2030)
4.4 Value Chain Analysis
4.5 Supply Chain Assessment
4.6 Regulatory Framework (FDA & CMS)
4.7 Impact of Digital Health & Remote Monitoring
4.8 Government & Federal Initiatives
(What this section provides: Macro and microeconomic forces, competitive intensity, pricing patterns, and regulatory environment influencing the US ICD market.)
5. Market Analysis – By Device Type
5.1 Overview
5.2 Segment Share Analysis (2025 & 2035)
5.2.1 Transvenous ICD
5.2.2 Subcutaneous ICD (S-ICD)
5.2.3 Cardiac Resynchronization Therapy Defibrillators (CRT-D)
(What this section provides: Product category performance trends, demand shifts, and technology adoption analysis.)
6. Market Analysis – By Indication
6.1 Overview
6.2 Segment Share Analysis (2025 & 2035)
6.2.1 Primary Prevention
6.2.2 Secondary Prevention
(What this section provides: Clinical usage trends and procedural demand forecasting based on indication.)
7. Market Analysis – By End-User
7.1 Overview
7.2 Segment Share Analysis (2025 & 2035)
7.2.1 Hospitals
7.2.2 Cardiac Specialty Centers
7.2.3 Ambulatory Surgical Centers
(What this section provides: End-user procurement patterns and procedural distribution analysis.)
8. Market Analysis – By Technology
8.1 Overview
8.2 Segment Share Analysis (2025 & 2035)
8.2.1 Single-Chamber ICD
8.2.2 Dual-Chamber ICD
8.2.3 MRI-Compatible ICD
8.2.4 Advanced & Next-Generation ICD Systems
(What this section provides: Technological advancements, MRI-conditional adoption, and innovation-driven growth insights.)
9. Market Analysis – By Geography
9.1 Introduction
9.2 Segment Share Analysis (2025 & 2035)
9.3 West Region
9.3.1 Regional Overview
9.3.2 Market Size & Forecast by State (2025–2035)
9.3.2.1 California
9.3.2.2 Washington
9.3.2.3 Colorado
9.3.2.4 Arizona
9.3.2.5 Oregon
9.3.2.6 Utah
9.3.2.7 Nevada
9.3.2.8 New Mexico
9.3.2.9 Idaho
9.3.2.10 Montana
9.3.2.11 Wyoming
9.3.2.12 Alaska
9.3.2.13 Hawaii
9.4 Northeast Region
9.4.1 Regional Overview
9.4.2 Market Size & Forecast by State (2025–2035)
9.4.2.1 New York
9.4.2.2 Massachusetts
9.4.2.3 New Jersey
9.4.2.4 Pennsylvania
9.4.2.5 Connecticut
9.4.2.6 Maine
9.4.2.7 Vermont
9.4.2.8 New Hampshire
9.4.2.9 Rhode Island
9.4.2.10 Delaware
9.5 South Region
9.5.1 Regional Overview
9.5.2 Market Size & Forecast by State (2025–2035)
9.5.2.1 Texas
9.5.2.2 Florida
9.5.2.3 Georgia
9.5.2.4 North Carolina
9.5.2.5 Tennessee
9.5.2.6 South Carolina
9.5.2.7 Alabama
9.5.2.8 Mississippi
9.5.2.9 Louisiana
9.5.2.10 Arkansas
9.5.2.11 Kentucky
9.5.2.12 Oklahoma
9.5.2.13 Maryland
9.5.2.14 Virginia
9.5.2.15 West Virginia
9.6 Midwest Region
9.6.1 Regional Overview
9.6.2 Market Size & Forecast by State (2025–2035)
9.6.2.1 Illinois
9.6.2.2 Ohio
9.6.2.3 Michigan
9.6.2.4 Minnesota
9.6.2.5 Indiana
9.6.2.6 Wisconsin
9.6.2.7 Missouri
9.6.2.8 Iowa
9.6.2.9 Kansas
9.6.2.10 Nebraska
9.6.2.11 North Dakota
9.6.2.12 South Dakota
(What this section provides: Detailed state-level demand trends, reimbursement environment analysis, and regional growth benchmarking across the US.)
10. Competitive Landscape & Company Profiles
10.1 Market Share Analysis (2025)
10.2 Competitive Positioning Matrix
10.3 Company Profiles
10.3.1 Medtronic
10.3.2 Abbott Laboratories
10.3.3 Boston Scientific Corporation
10.3.4 Biotronik SE & Co. KG
10.3.5 MicroPort CRM
10.3.6 LivaNova PLC
10.3.7 ZOLL Medical Corporation
10.3.8 Stryker Corporation
10.3.9 Cook Medical
10.3.10 Osypka Medical
10.3.11 Cardiac Science
10.3.12 BPL Medical Technologies
10.3.13 Cardiovascular Systems Inc.
10.3.14 Edwards Lifesciences
10.3.15 Philips Healthcare
10.3.16 GE HealthCare
10.3.17 Siemens Healthineers
10.3.18 EBR Systems
10.3.19 Lepu Medical
10.3.20 Integer Holdings Corporation
10.3.21 Cirtec Medical
10.3.22 Spectrum Plastics Group
10.3.23 Merit Medical Systems
10.3.24 Other Emerging Players
(What this section provides: Competitive benchmarking, innovation strategy comparison, financial overview, and US positioning of major ICD manufacturers.)
11. Future Market Outlook (2025–2035)
11.1 Scenario-Based Forecasting
11.2 Technological Disruption Outlook
11.3 Emerging Clinical Trends
11.4 Investment & Expansion Opportunities
(What this section provides: Forward-looking strategic insights for investors, manufacturers, and healthcare providers.)
12. Strategic Recommendations
(What this section provides: Actionable insights and growth strategies for market participants.)
13. Disclaimer
(What this section provides: Legal clarifications, assumptions, and usage limitations.)
List of Tables
TABLE 1: List of Data Sources
TABLE 2: Market Drivers; Impact Analysis
TABLE 3: Market Restraints; Impact Analysis
TABLE 4: US Implantable Cardioverter Defibrillator Devices Market: Device Type Snapshot (2025)
TABLE 5: Segment Dashboard; Definition and Scope, by Device Type
TABLE 6: US Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 7: US Implantable Cardioverter Defibrillator Devices Market: Indication Snapshot (2025)
TABLE 8: Segment Dashboard; Definition and Scope, by Indication
TABLE 9: US Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 10: US Implantable Cardioverter Defibrillator Devices Market: End-User Snapshot (2025)
TABLE 11: Segment Dashboard; Definition and Scope, by End-User
TABLE 12: US Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 13: US Implantable Cardioverter Defibrillator Devices Market: Technology Snapshot (2025)
TABLE 14: Segment Dashboard; Definition and Scope, by Technology
TABLE 15: US Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 16: US Implantable Cardioverter Defibrillator Devices Market: Regional Snapshot (2025)
TABLE 17: Segment Dashboard; Definition and Scope, by Region
TABLE 18: US Implantable Cardioverter Defibrillator Devices Market, by Region, 2022–2035 (USD Million)
TABLE 19: California Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 20: California Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 21: California Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 22: California Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 23: Washington Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 24: Washington Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 25: Washington Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 26: Washington Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 27: Colorado Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 28: Colorado Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 29: Colorado Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 30: Colorado Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 31: Arizona Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 32: Arizona Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 33: Arizona Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 34: Arizona Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 35: Oregon Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 36: Oregon Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 37: Oregon Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 38: Oregon Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 39: West – Others Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 40: West – Others Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 41: West – Others Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 42: West – Others Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
(Nevada, Utah, New Mexico, Idaho, Montana, Wyoming, Alaska, Hawaii)
TABLE 43: New York Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 44: New York Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 45: New York Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 46: New York Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 47: Massachusetts Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 48: Massachusetts Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 49: Massachusetts Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 50: Massachusetts Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 51: New Jersey Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 52: New Jersey Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 53: New Jersey Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 54: New Jersey Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 55: Pennsylvania Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 56: Pennsylvania Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 57: Pennsylvania Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 58: Pennsylvania Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 59: Connecticut Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 60: Connecticut Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 61: Connecticut Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 62: Connecticut Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 63: Northeast – Others Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 64: Northeast – Others Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 65: Northeast – Others Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 66: Northeast – Others Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
(Maine, Vermont, New Hampshire, Rhode Island, Delaware)
TABLE 67: Texas Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 68: Texas Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 69: Texas Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 70: Texas Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 71: Florida Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 72: Florida Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 73: Florida Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 74: Florida Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 75: Georgia Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 76: Georgia Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 77: Georgia Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 78: Georgia Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 79: North Carolina Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 80: North Carolina Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 81: North Carolina Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 82: North Carolina Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 83: Tennessee Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 84: Tennessee Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 85: Tennessee Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 86: Tennessee Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 87: South Carolina Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 88: South Carolina Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 89: South Carolina Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 90: South Carolina Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 91: Alabama Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 92: Alabama Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 93: Alabama Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 94: Alabama Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 95: South – Others Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 96: South – Others Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 97: South – Others Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 98: South – Others Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
(Mississippi, Louisiana, Arkansas, Kentucky, Oklahoma, Virginia, Maryland, West Virginia)
TABLE 99: Illinois Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 100: Illinois Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 101: Illinois Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 102: Illinois Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 103: Ohio Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 104: Ohio Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 105: Ohio Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 106: Ohio Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 107: Michigan Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 108: Michigan Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 109: Michigan Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 110: Michigan Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 111: Minnesota Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 112: Minnesota Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 113: Minnesota Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 114: Minnesota Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 115: Indiana Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 116: Indiana Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 117: Indiana Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 118: Indiana Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 119: Wisconsin Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 120: Wisconsin Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 121: Wisconsin Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 122: Wisconsin Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 123: Missouri Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 124: Missouri Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 125: Missouri Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 126: Missouri Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
TABLE 127: Midwest – Others Implantable Cardioverter Defibrillator Devices Market, by Device Type, 2022–2035 (USD Million)
TABLE 128: Midwest – Others Implantable Cardioverter Defibrillator Devices Market, by Indication, 2022–2035 (USD Million)
TABLE 129: Midwest – Others Implantable Cardioverter Defibrillator Devices Market, by Technology, 2022–2035 (USD Million)
TABLE 130: Midwest – Others Implantable Cardioverter Defibrillator Devices Market, by End-User, 2022–2035 (USD Million)
(Iowa, Kansas, Nebraska, North Dakota, South Dakota)
List of Figures
FIGURE 1: US Implantable Cardioverter Defibrillator Devices Market Segmentation
FIGURE 2: Market Research Methodology
FIGURE 3: Value Chain Analysis
FIGURE 4: PESTLE Analysis
FIGURE 5: Porter’s Five Forces Analysis
FIGURE 6: Market Attractiveness Analysis
FIGURE 7: Market Dynamics
FIGURE 8: Innovation & Patent Landscape (2020–2025)
FIGURE 9: Competitive Landscape; Key Company Market Share Analysis, 2025
FIGURE 10: Device Type Segment Market Share Analysis, 2025 & 2035
FIGURE 11: Device Type Segment Market Size Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 12: Indication Segment Market Share Analysis, 2025 & 2035
FIGURE 13: Indication Segment Market Size Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 14: End-User Segment Market Share Analysis, 2025 & 2035
FIGURE 15: End-User Segment Market Size Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 16: Technology Segment Market Share Analysis, 2025 & 2035
FIGURE 17: Technology Segment Market Size Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 18: Regional Segment Market Share Analysis, 2025 & 2035
FIGURE 19: Regional Segment Market Size Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 20: West Region US Implantable Cardioverter Defibrillator Devices Market Share and Leading Players, 2025
FIGURE 21: Northeast Region US Implantable Cardioverter Defibrillator Devices Market Share and Leading Players, 2025
FIGURE 22: South Region US Implantable Cardioverter Defibrillator Devices Market Share and Leading Players, 2025
FIGURE 23: Midwest Region US Implantable Cardioverter Defibrillator Devices Market Share and Leading Players, 2025
FIGURE 24: West Region Market Share Analysis by State, 2025
FIGURE 25: California Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 26: Washington Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 27: Colorado Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 28: Arizona Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 29: Oregon Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 30: Others (West Region) Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 31: South Region Market Share Analysis by State, 2025
FIGURE 32: Texas Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 33: Florida Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 34: Georgia Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 35: North Carolina Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 36: Tennessee Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 37: South Carolina Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 38: Alabama Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 39: Others (South Region) Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 40: Midwest Region Market Share Analysis by State, 2025
FIGURE 41: Illinois Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 42: Ohio Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 43: Michigan Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 44: Minnesota Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 45: Indiana Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 46: Wisconsin Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 47: Missouri Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 48: Others (Midwest Region) Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 49: Northeast Region Market Share Analysis by State, 2025
FIGURE 50: New York Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 51: Massachusetts Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 52: New Jersey Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 53: Pennsylvania Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 54: Connecticut Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)
FIGURE 55: Others (Northeast Region) Implantable Cardioverter Defibrillator Devices Market Size, Forecast and Trend Analysis, 2025–2035 (USD Million)